JPEC Introduction
Joint Penang Independent Ethics Committee (JPEC) is an ethics committee established in 2002 under the joint authority of RCSI & UCD Malaysia Campus and Info Kinetic / Clinical Research Centre, Penang.
JPEC conducts initial and continuing ethical review of clinical trials conducted at private medical institutions in Penang. JPEC is unique in that while most ethics committees are institutions i.e. formed by and comprising of members from one particular institution, JPEC unites members from private and public medical institutions from Penang, Malaysia.


Main Objective
To protect trial subject welfare, rights and safety while taking into consideration the risks and benefits of trials.
JPEC is aware of the need for well-designed scientific trials for medical progress.



JPEC complies with the requirements of the Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects and ICH Good Clinical Practice as well as the Malaysian GCP.
JPEC has written Standard Operating Procedures (SOPs) as well as performance markers that guide JPEC’s continuous improvement process. JPEC’s SOPs being reviewed regularly and it incorporate the WHO Operating Guidelines for Ethics Committees that Review Biomedical Research 2000.
JPEC is officially registered (Registration #: IORG0006175) with the Office of Human Research Protection (OHRP) in USA. Registration with OHRP enables JPEC to conduct ethical review of studies that are funded by the USA National Institute of Health (NIH), one of the largest and most widely known research funders in America.
JPEC is one of the ethics committees in Malaysia that is registered with the Drug Control Authority (DCA), an authority established for the purpose of regulating the Control of Drugs and Cosmetics Regulations, 1984 and undergoes regular GCP audit inspection from the National Pharmaceutical Control Bureau (NPCB).
JPEC Members
JPEC has 3 laypersons in its committee – the lawyer, accounting and a corporate field. They provide essential views, which reflect those of potential subjects in clinical trials. The majority of members are doctors from various specialties, thus enriching the review expertise within the committee. An important strength of a joint committee like JPEC is that the majority of members are not affiliated to the authority under which JPEC is formed. This enables independent decision making. Only 3 out of 11 members is affiliated to the parent authorities.
JPEC Secretariat
The JPEC Secretariat is the administrative unit of JPEC and is responsible for the following duties:
- Giving advice and information regarding rules and regulation in ethical aspects through communication with the JPEC members to the investigator.
- Communicate all decisions of JPEC to the applicant.
- In charge of compiling safety reports from clinical studies sponsors to Joint Penang Independent Ethics Committee (JPEC).
- In charge of incoming and outgoing correspondences.
- Ensure quality requirements of GCP are met.
- Organise JPEC meetings.
Secretary (non-voting member):
Ms Siti Nur Afiqah
JPEC Secretariat
Joint Penang Independent Ethics Committee
c/o Clinical Research Centre
4, Jalan Sepoy Lines,
10450 George Town,
Penang, Malaysia.
The JPEC Secretariat c/o Clinical Research Centre
4, Jalan Sepoy Lines, 10450 George Town, Penang.
04-228 7272
New Application Procedure
Original
- Study Protocol
- Subject Information Sheet and Informed Consent Form
- Advertisements, Questionnaire
- Investigator’s Brochure or Product Summary
- Investigator(s)’ Curriculum Vitae, GCP Certificates, APC
- Recruitment Material
- Insurance Coverage for subjects in event of trial-related injury
- Statement from sponsor indemnifying the relevant staff, investigator, institution and JPEC
Two Full Dossiers
- Study Protocol
- Subject Information Sheet and Informed Consent Form
- Advertisements, Questionnaire
- Investigator’s Brochure or Product Summary
- Investigator(s)’ Curriculum Vitae, GCP Certificates, APC
- Recruitment Material
- Insurance Coverage for subjects in event of trial-related injury
- Statement from sponsor indemnifying the relevant staff, investigator, institution and JPEC
10 Simplified Dossiers
- Study Protocol
- Subject Information Sheet and Informed Consent Form
- Advertisements, Questionnaire
- Investigator(s)’ Curriculum Vitae, GCP Certificates, APC
- Recruitment Material
- Insurance Coverage for subjects in event of trial-related injury
- Statement from sponsor indemnifying the relevant staff, investigator, institution and JPEC
- Submit 13 copies (1 original and 12 photocopies =2 full dossiers+ 10 simplified dossiers) of the above supporting documents.
- Investigators that will conduct an interventional clinical trial should be trained and certified in an approved training of Good Clinical Practice (GCP) by the National Committee for Clinical Research (NCCR).
- All new applications are to be submitted with a one-off application fee of RM2,000 per research project made payable to ‘RCSI & UCD Malaysia Campus’. Application(s) will only be processed upon receipt of full payment.
- Application fees are as follows:
- BA / BE (Bio-availability / Bio-equivalence) study : RM 4000/ per study.
- Industry-sponsored study: RM 5000/ per study.
- Kindly refer the following for the bank-in details:Beneficiary Name: PENANG MEDICAL COLLEGE SDN BHDBank Name: UNITED OVERSEAS BANK (M) BHD.Virtual Account No: 10542-9100-008Swift Code: UOVBMYKLBranch Name: UOBM Jalan Kelawei
Branch Address: 9, Jalan Kelawei, 10250 Pulau Pinang, Malaysia
- It is the prerogative of JPEC to waive this fee for investigator-driven research, which does not receive funding.
- Any incomplete applications will lead to a delay in review.
- Applicants are required to register all research projects with the National Medical Research Register (NMRR) at https://www.nmrr.gov.my/fwbLoginPage.jsp. The full NMRR ID should be provided to JPEC for trials seeking approval from JPEC. No approval will be given without the NMRR ID.
Amendment Application Procedure
- Complete the ‘Application for Amendment to Research Project involving Human Subjects’(signed by PI or CI for multiple sites) form and submit one original copy with relevant supporting documents. For applications, that may affect subject safety, rights or welfare, the proposed amendments and study documents(s) with proposed amendments clearly indicated (tracked changes) and the rationale for the amendment needs to be submitted along the with the form.
- These applications may either undergo an expedited review or a meeting review:
Meeting Review
Amendments affecting the subject’s safety, right or welfare.
Expedited Review
Amendments are purely administrative or logistical or clearly do not affect subject safety, rights or welfare.
Sometimes minor administrative amendments are submitted to JPEC for its information only. These include amendments involving logistical or admirative aspects of the trial. (e.g.: change of monitor(s), telephone number(s), change of address of central laboratory etc.) The JPEC officer will review and acknowledge receipt of the information on such amendments.
Forms required for submission with supporting documents
Application form to conduct a new research project involving human subjects.
Applicant’s Document Checklist for submitting an Application to conduct a new research project.
Applicant’s checklist of minimum elements in the informed consent form and written subject information.
Other Forms required for monitoring of the trials approved by JPEC
For SAE Reporting
For reporting of study closure
For annual reporting for trial progress
For Amendment Application
For BA/BE renewal
What does JPEC look for in its review of application?
01
Scientific design and conduct of study
02
Recruitment of subjects
03
Protection of subject confidentiality
04
Informed consert process
05
Community considerations
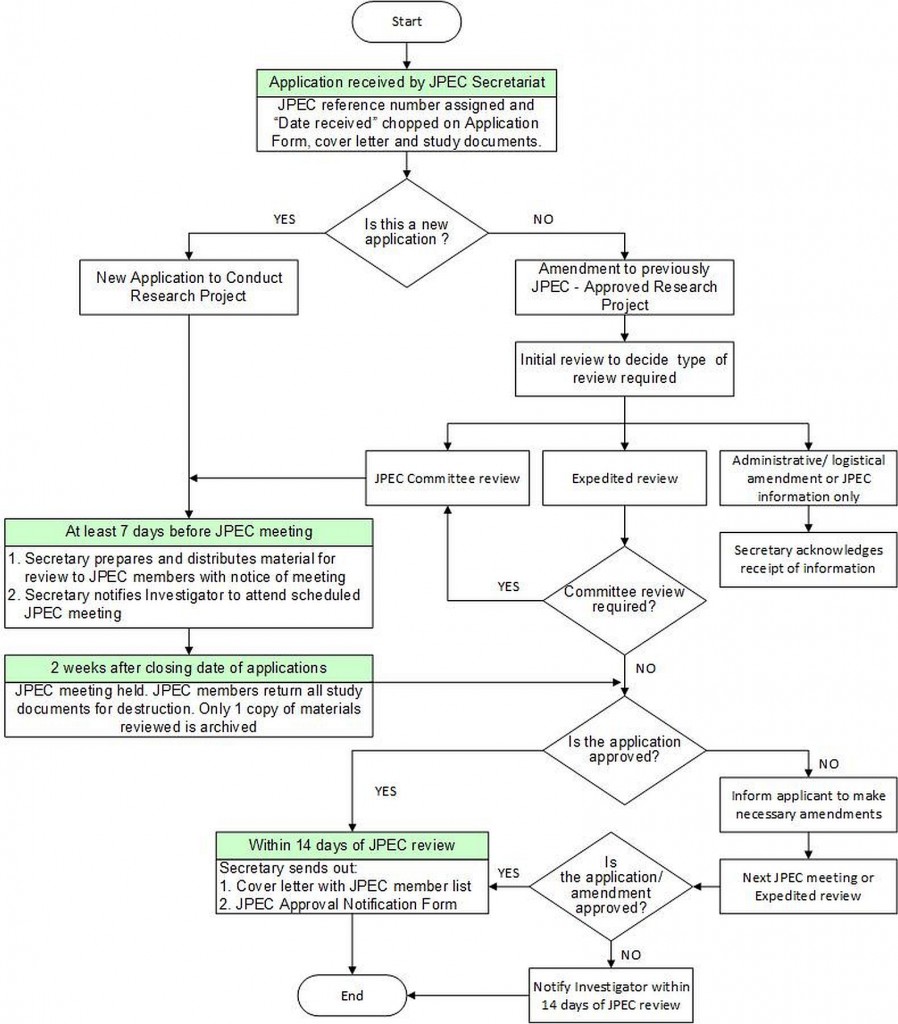
- JPEC informs applicants of its decision in writing within 14 days from its review. In cases of a full approval, a statement of responsibilities of the investigator is provided. In cases of negative decisions (i.e rejected, termination), reasons are provided.
- JPEC approvals for new research projects are valid for 12 months from the date of issue of the JPEC Decision Notification Form. A new application to JPEC will have to be submitted for research projects that are not commenced within 12 months from the date of issue of the JPEC Decision Notification Form.
- In some instances, JPEC grants conditional approvals. In these cases applicants must show evidence that they have complied with these conditions within 8 weeks from this notification to receive a full approval. After that date, the file on this application will be closed if no response is received by JPEC. A new application will need to be submitted if the applicant is keen on pursuing ethical approval.
All adverse drug reactions (ADRs), which are both serious and/or unexpected, are to be reported to JPEC. In the case of serious adverse events ( SAEs ) occurring in the investigator’s institution, the investigator is to notify the JPEC Chairperson within 24 hours of its notification to the investigator, using the JPEC Serious Adverse Event Report.
For SAEs outside of the investigator’s institution (applicable in multicentre trials):
- Only Suspected, Unexpected and Serious Adverse Reaction (SUSAR) are to be submitted to JPEC
- Those that are fatal/life-threatening : Investigator is to report (cc. local investigator) the SUSAR to JPEC within 7 calendar days from first knowledge by the INVESTIGATOR. If there is additional information, this should be submitted within 8 calendar days.
- Those that are non-fatal/life-threatening SUSARs, to submit to JPEC within 15 calendar days from first knowledge by the INVESTIGATOR.
- Only protocol specific investigational product(s) SUSARs are to be submitted to JPEC.
- All periodic SUSAR reporting (line listing) are to be accompanied by a brief report by sponsor highlighting main points of concern, if any.
- Annual Safety Report is expected on an annual basis throughout the whole clinical trial course. This report will describe concisely all new safety information relevant for the trial and to assess the safety conditions of subjects in the trial.
After JPEC has issued ethical approval for a trial to begin, it continues to monitor the trial to ensure that the subjects’ welfare, safety and rights are protected through the dynamics of time. This is done by receiving notification from investigators of the following:
- Amendments to the trial
- Reports – serious adverse event, trial progress and closure reports
- Or by conducting an audit of the trial
A Research Project Progress Report is to be submitted to JPEC at specified intervals (usually annually) once a trial is ongoing. Upon study closure, a Research Project Closure Report is to be submitted to JPEC. Normally, the first research project progress report is due one year from the date of issue of the JPEC Decision Notification Form. However, studies with higher risk to subjects may require more frequent progress reporting. The Research Progress report is not applicable to short studies, which are closed within a year from date of issue of the Decision Notification Form. In this instance, only the study closure report needs to be submitted o JPEC.
Investigator(s) involved in ongoing trial(s) are required to submit to JPEC their most recent, updated Curriculum Vitae and Annual Practicing Certificate annually (beginning of each year) until JPEC is notified of study or site closure.
If the applicant perceives a request for amendment, substantial change, resubmission or non approval unfair or unwarranted, the applicant will initially confer with the Secretary for clarification of the reasoning of JPEC.
If, after consultation with the Secretary, the applicant is not satisfied, the applicant has the right to request, and JPEC is obligated to provide reconsideration of negative decision through an appeal process.
Only appeals that are made in writing will be considered. Letters of appeal must be received within 30 days in advance of the next scheduled JPEC meeting.
The appeal process would involve four steps
- A discussion of the issue appealed
- A review of the evidence
- A considered decision arrived at
- Communication of the decision outcome made during a convened meeting
JPEC may invite the appellant to attend the meeting
The decision of the appeal is based on
- A full and clear understanding of the facts of the original review
- The nature of the appeal
- Legal opinion
- Adherence to the ICH and Malaysian Guideline for GCP
- Adherence to JPEC SOPs
- Attention to the internal and external reputation of Penang Medical College and Info Kinetics Sdn Bhd Clinical Research Centre
The outcomes to appeal would include
- Whether the appeal is successful or not
- Reasons for the decision
- Communication of the decision is within 7 days of the meeting
- JPEC’s decision is final
A simplified dossier is similar to that of the full dossier except the simplified dossier would not need to include the following documents:
- Investigator Brochure
- Insurance Policy
- Application Form
For a single protocol with multiple sites, only one application form is needed for the initial application and also application for amendment and need to be signed by the Corresponding PI. For addition of sites to an already approved protocol, a copy of the Principal Investigator’s CV, Annual Practicing Certificate and GCP certificate would need to be submitted along with the site specific documents.
Some of the common conditions for approval for approval not met are: i) ICF does not contain the applicable requirements according to the Applicant’s checklist of minimum requirements in the informed consent form and written subject information. ii) Doctor’s name advertised in patient recruitment advertisements and materials iii) Incomplete document submitted (i.e no indemnity letter) iv) The coverage of medical fees through healthcare insurance provider in the informed consent form which is not applicable in Malaysia v) Principal Investigator is not certified in Good Clinical Practice for interventional clinical trials. vi) ICF are in mixed languages
Documents (besides the protocol and Investigator Brochure) that are not distributed to patients and are not source documents need not to be submitted to JPEC as JPEC do not review documents those documents. However, the applicant is still allowed to submit a copy of those documents (i.e JPEC acknowledges the submission) but an approval will not be issued as it did not undergo a review.
Yes. You only need to report related and unrelated SAEs of sites approved by JPEC only but SUSARs from all over the world. Reporting is done specific to the research protocol.
For serious adverse events (SAEs) occurring in the investigator’s institution, the investigator is to notify the JPEC Chairperson within 24 hours of its notification to the investigator. Investigator is to report (cc. local investigator) the fatal/life-threatening SUSAR to JPEC within 7 calendar days from first knowledge. If there is additional information, this should be submitted within 8 calendar days. Those that are non-fatal/life-threatening SUSARs, to submit to JPEC within 15 calendar days from first knowledge.
There is no timeline for the submission of SUSAR line listings. However, all applicants are required to submit the line listing whenever one is available from the sponsor. All periodic SUSAR reporting (line listing) are to be accompanied by a brief report by sponsor highlighting main points of concern, if any.
Protocol deviation involving JPEC approved sites needs to be reported through a cover letter signed by the Investigator.
JPEC shall give an opinion within a maximum of 35 days of the date of receipt of the valid and completed proposed amendment submitted.
JPEC requires to be indemnified as JPEC is not affiliated under any institution and on its own is an independent body therefore is not indemnified against claims arising from the trial, except for claims that arise from malpractice and/or negligence. JPEC can be indemnified in a separate indemnity letter or in a paragraph in the CTA agreement.
The JPEC approval is only valid for 12 months from the date of the approval letter issued.
Yes upon completion of your study, you are required to send in a Closure Report Form to JPEC within 90 days of scheduled study closure.
Yes upon termination of your study, you are required to send in a Closure Report to JPEC within within 15 days with reasons for suspension/termination and a summary of results provided.
Date Of Meeting
- MO
- TU
- WE
- TH
- FR
- SA
- SU
- 24
- 25
- 26
- 27
- 28
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 1
- 2
- 3
- 4
- 5
- 6



